FlowMIR®, Disposable Turbine
Disposable, factory-calibrated turbine flowmeter, individually packaged with cardboard mouthpiece.
-
-
Unaffected by environmental conditions
-
Reliable and accurate measurement
-
Hygienic
FlowMIR® turbines are patented single-use devices by MIR – Medical International Research, specifically designed to ensure accurate, reliable, and safe spirometry measurements.
Ideal for maximum hygiene, these disposable turbines are the perfect solution for those seeking simplicity and convenience. Factory-calibrated and ready to use, they eliminate the need for further calibration, cleaning, or maintenance.
The integrated cardboard mouthpiece makes them even easier to handle, ensuring immediate usability in any setting. The turbines prevent direct contact between patient and device, and between different patients, minimizing the risk of cross-contamination.
While an antibacterial filter is not required due to their disposable nature, local regulations may vary and in some countries, its use may be mandatory to comply with safety standards.
Clinical Evidence
1) Published in 2023
“Comparison of Pneumotachometer and Portable Digital Turbine Spirometry for Field-Based Assessment: An Air Quality, Environment, and Respiratory Outcomes in Bronchopulmonary Dysplasia Study”
This study compared a MIR spirometer with a pneumotachograph. It concluded:
“The mean difference in FEV1 between the Pneumotrac and the MIR spirometer was 0.079 L (with 95% limits of agreement ranging from −0.141 to 0.298 L), and for FVC it was 0.075 L (with 95% limits of agreement from −0.171 to 0.322 L). These differences were relatively small and did not indicate any systematic or volume-dependent bias.”
The study concluded:
“Turbine spirometers may represent a promising and practical solution for conducting field-based pulmonary function tests in children.”
2) Published in 2020
“Comparison of a Portable Smart Spirometer Against Two Lab-Based Desktop Systems”
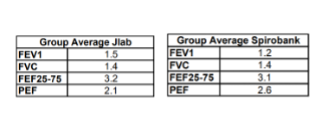
This study validated the Spirobank® smart spirometer with FlowMIR® turbine against two lab systems:
- NDD Easy on-PC
- Jaeger Masterscreen PFT
Conducted within the NHS Greater Glasgow and Clyde, the study confirmed that Spirobank® smart produced clinically acceptable results, comparable to lab systems. The device proved useful for home monitoring, especially during the COVID-19 pandemic when hospital visits were limited.
The conclusion stated that the intra-test coefficient of variation for Spirobank® smart was clinically acceptable and not significantly different from the other tested devices, confirming that MIR turbines support accurate and reliable measurements—even under remote supervision by healthcare professionals.
Compatibility
FlowMIR® is compatible with Minispir, Minispir Light, Spirolab, Spirodoc, Spirobank II Smart, Spirobank II Advanced, Spirobank II Basic.
Product Code: 910004
Also available in dispenser boxes of 10 (Code: 91000410) or 60 units (Code: 91000460).
All the features
-
Compliance with International Guidelines
FlowMIR® fully complies with the ATS/ERS (American Thoracic Society / European Respiratory Society) guidelines for spirometry testing, ensuring accurate measurement of FVC, FEV1, PEF, and other key lung function parameters.
-
Hygienic
A rigid plastic tube creates a physical barrier between the exhaled air and the sensing system, ensuring maximum hygiene.
-
Unaffected by Environmental Conditions
Turbines are unaffected by temperature, pressure, or humidity, ensuring consistent performance in any environment.
-
Mechanical Simplicity
The turbine’s mechanical design eliminates the need for complex electronics, reducing potential sources of error and drift.
-
Disposable
Each turbine includes an integrated mouthpiece and is individually packaged, sterile, and ready to use—eliminating the need for disinfection and streamlining workflow.
-
High Accuracy, No Calibration Needed
Thanks to individual quality control, FlowMIR® delivers consistent and reliable performance. No calibration or maintenance is required, reducing setup time and error margins.
-
Reliability and Accuracy
Over the past 30 years, MIR has developed sophisticated algorithms to correct turbine inertia, enabling dynamic compensation of the turbine’s mechanical behavior.
Thanks to these technologies, MIR turbines ensure high accuracy even at low flows and rapid flow increases, meeting compliance standards:- ATS PW24 and FT26 curves
- ISO 23747 and ISO 26782
Scientific studies show that MIR turbine spirometers provide clinically comparable results to lab-based instruments, including in pediatric populations.
-
High Sampling Rate and Precision
MIR turbines use a highly sophisticated algorithm to build the Volume/Time curve with interpolation times no greater than 10 mS (100Hz). At high flow rates, sampling intervals can be under 1mS.
Become a Dealer
Interested in Becoming a Dealer? Learn more on the requirements and how to become an official MIR dealer.
Infection control

MIR turbines overview

ATS / ERS |

Reusable Turbine Flowmeter
|

FlowMIR®, Disposable Turbine
|

Single Patient Reusable Turbine and Mouthpiece
|
|---|---|---|---|
|
ATS / ERS |

Reusable Turbine Flowmeter
|

FlowMIR®, Disposable Turbine
|

Single Patient Reusable Turbine and Mouthpiece
|
|
Compatible Spirometers |
|||
|
Mouthpiece |
To be purchased separately
|
Included
|
Included (reusable)
|
|
Antiviral Filter |
To be purchased separately
|
Not required
|
Not required
|
|
Turbine Disinfection |
Required
|
Not required
|
Required (soapy water)
|
|
Turbine Calibration |
Not required but available via software at any time
|
Not required but available via software at any time
|
Not required
|
|
Packaging |
Carton box
|
Individually sealed in carton dispenser (60pcs or 10pcs)
|
Individually sealed in carton box
|
|
Metal mesh |
Included
|
Not required
|
Included
|
|
Turbine Lifetime |
5 years
|
Single use (no expiration date)
|
1 year
|
|
Maintenance Cost |
High
|
None
|
Low
|
Looking for our Company and Product Certificates and Compliance Documents?
Ask for information or support
If you need more information about the product or you need some help, fill the form.